Nephron Physiology
 |
| source |
 |
sourceKey Points:Early Proximal Tubule: reabsorb all glucose, amino acids and most of bicarbonate, sodium, chloride, phosphate and water. Generates and secretes ammonia.Thin descending loop of Henle: passive reabsorbs water. Think Ascending Loop of Henle: actively reabsorbs Na+, K+ and Cl-. Responsible for paracellular reabsorption of Mg2+ and Ca2+. Impermeable to H2O. Early Distal Convoluted Tubule: actively reabsorb Na_ and Cl-. Collecting Tubule: reabsorb Na+ by secreting K+ and H+ using aldosterone. Reabsorb water by using ADH. PTH:1) at the PCT = inhibits Na+/Phosphate cotransport and promotes phosphate excretion 2) at the Early distal convoluted tubule = increase Ca2+/Na+ exchange and promotes Ca2_ reabsorption
Relative Concentrations along proximal tubule
Renin -angiotensin-aldosterone system
juxtaglomerular apparatus
|
1) Macula Densa
source
2) Juxtaglomerular Cells
Enlarged, smooth muscle cells that have secretory granules containing renin. These act on mechanoreceptors.
OVERVIEW:
3) Mesanglial Cells
Have phagocytic and contractile properties and also influences capillary filtration.
Angiotensin ll influences:
1) Baroreceptors: Baroreceptor reflex influences the GFR via long term regulation of arterial blood pressure
1. Higher arterial blood pressure triggers both long term and short term changes to lower blood pressure.
2. High blood pressure sensed in aortic arch and carotid sinus baroreceptors
3. These release vasoactive chemicals that increase sympathetic activity and promote generalized arteriolar constriction such as ATP, endothelin/ADH/vasopressin, and adenosine
4. The increased sympathetic activity increases cardiac output
5. Increased sympathetic activity also increases general vasoconstriction - leading to higher total peripheral resistance and afferent arteriolar vasoconstriction
6. Thus, glomerular capillary blood pressure goes down,
7. GFR down
8. Urine volume down
9. More salt and fluid are conserved
10. These go back to increase blood pressure
Thus, low blood pressure promotes long and short term adjustment. Short term is the increased sympathetic activity and vasoconstriction effects to increase cardiac output and total peripheral resistance. Long term is the vasoconstriction of afferent arterioles to glomerulus, lowering GFR, lowering urine volume and retaining fluid and salt to increase blood pressure.
2) AT1 RECEPTOR: vascular smooth muscle vasoconstriction = increase BP

3) ALDOSTERONE: Adrenal gland releases aldosterone.
 |
| SOURCE |
 |
| SOURCE |
Kidney Endocrine Functions:
1) Erythropoietin released by interstitial cells of the peritubular capillary due to hypoxia.
 |
| source |
 |
source
3) Renin
Triggers to release renin: decreased BP, low sodium level (macula densa) and increased sympathetics.
|
4) Prostaglandins

Hormones acting on kidney
1) artial natiruetic peptide (ANP)
detects increases in BP and responds by prompting the kidney to keep as much H2O as possible in the filtrate. Mechanism: decreasing NaCl reabsorption and therefore decreasing H2O (b/c it follows sodium)
5) ADH
Potassium shifts
paracrine secretion vasodilates afferent arterioles. Effect can be inhibited by NSAIDs which then lead to acute renal failure.

 |
| source |
Hormones acting on kidney
1) artial natiruetic peptide (ANP)
detects increases in BP and responds by prompting the kidney to keep as much H2O as possible in the filtrate. Mechanism: decreasing NaCl reabsorption and therefore decreasing H2O (b/c it follows sodium)
2) Parathyroid Hormone (PTH)
low plasma calcium, high plasma phosphate or low plasma 1,25-(OH)2 vitamin D ===> increases concentration of calcium resorption in DCT, decreases phosphate reabsorption in the PCT, increase 1,25-(OH)2 vitamin D production in the kidney
PTH increases calcium reabsorption in DCT
 |
| source |
PTH decreases phosphate reabsorption in PCT
 |
| source |
3) Angiotensin ll (AT ll)
decreased BP ==> efferent arteriole constriction ==> increase GFR and FF (FF = GFR/RPF)
- Angiotensin II stimulates Na+/H+ exchangers located on the apical membranes (faces the tubular lumen) of cells in the proximal tubule and thick ascending limb of the loop of Henle in addition to Na+ channels in the collecting ducts. This will ultimately lead to increased sodium reabsorption
4) Aldosterone
decrease blood volume + increased plasma osmolarity (K+) ===> aldosterone secretion ==> increased Na+ resorption with K+ and H+ secretion
 |
| source |
 |
| source |
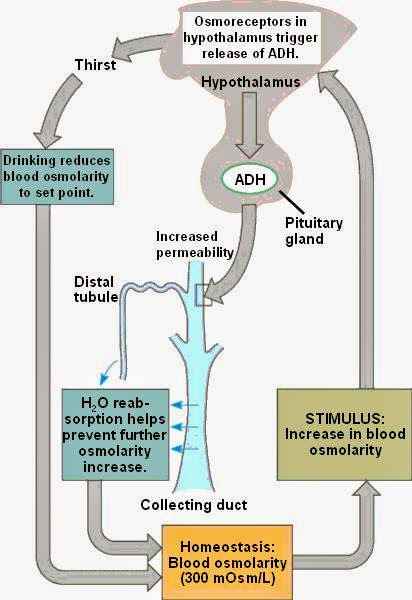
increased plasma osmolarity + decreased blood volume ==> ADH secretion ==> ADH bind to principal cells' receptors ==> increase aquaporins ==> increase H2O absorption
 |
| source |
Shifts K+ out of cell (causing hyperkalemia): DO Insulin LAB
1. Digitalis
 |
| source digitalis inhibits the Na-K ATPase (which pumps 3 Na out of the cell and 2 K in); as such, it can result in hyperkalemia and a variety of cardiac arrhythmias. |
2. HyperOsmolarity
hyperosmolarity induces water efflux out of cells, and by solvent drag increases intravascular potassium concentrations. Drugs such as mannitol can therefore cause translocational hyperkalemia.
3. Insulin deficiency
Ingested K+ is absorbed rapidly and enters the portal circulation, where it stimulates insulin secretion. Insulin increases Na+,K+-ATPase activity and facilitates potassium entry into cells, thereby averting hyperkalemia.
4. Lysis of cells
When there is a rapid amount of cellular destruction, the components of the cells (including potassium), will move outside of the cell, and into the blood stream.
5. Acidosis
 |
| source |
Normally, agonist binding to the beta2-adrenergic receptor stimulates the formation of cyclic AMP, which acts through protein kinase A to phosphorylate and activate the Na-K-ATPase pump, leading to the influx of potassium into cells. Competitive inhibition of the beta2 receptor by beta blockers decreases Na-K-ATPase function and reduces potassium uptake by cells.
 |
| source |
Shifts K+ Into Cell (causing Hypokalemia) Insulin shifts K+ into cells
1) Hypo-osmolarity
2) Insulin
3) Alkalosis
 |
| source |
4) B-adrenergic agonist
Electrolyte disturbances
LOW SERUM CONCENTRATION:
1) Na+ : nausea, malaise, stupor, coma
hyponatremia
2) K+ : U waves on ECG, flattened T waves, arrhythmias, muscle weakness
3) Ca2+ : Tetany, seizures
4) Mg2+ : Tetany, arrhythmias
5) PO43- : bone loss, osteomalacia (bone softening)
4) Mg2+
hypermagnesemia
decreased DTRs, lethargy, bradycardia, hypotension, cardiac arrest, hypocalcemia (Mg2+ inhibits calcium from being released from the sarcoplasmic reticulum)
5) PO43-
Renal stones, metastatic calcifications, hypocalcemia
causes: airway obstruction, acute lung disease, Chronic lung disease, Opioids, sedatives, weakening respiratory muscles
Metabolic acidosis with compensation (hyperventilation) pH < 7.4, PCo2 < 40 mmHg
1) increased anion gap
MUDPILES
Methanol (formic acid)
Uremia = is the illness accompanying kidney failure (also called renal failure), in particular the nitrogenous waste products associated with the failure of this organ.
Diabetic ketoacidosis = develops when your body is unable to produce enough insulin. Insulin normally plays a key role in helping sugar (glucose) — a major source of energy for your muscles and other tissues — enter your cells. Without enough insulin, your body begins to break down fat as an alternate fuel. This process produces a buildup of toxic acids in the bloodstream called ketones, eventually leading to diabetic ketoacidosis if untreated.
Propylene glycol
Iron tablets
Lactic acidosis - The condition typically occurs when cells receive too little oxygen (hypoxia), for example, during vigorous exercise. In this situation, impaired cellular respiration leads to lower pH levels. Simultaneously, cells are forced to metabolize glucose anaerobically, which leads to lactate formation. Therefore, elevated lactate is indicative of tissue hypoxia, hypo perfusion and possible damage.
Renal Tubular Acidosis - is a disease that occurs when the kidneys fail to excrete acids into the urine, which causes a person's blood to remain too acidic.
Type 1 ("distal"): collecting tubule can't excrete H+, hypokalemia (K+/H+ exchanger), risk for calcium phosphate kidney stones
Type 2 ("proximal"): proximal tubule can't reabsorb HCO3-. hypokalemia. Seen in Fanconi's syndrome.
hypokalemia
 |
| source |
3) Ca2+ : Tetany, seizures
hypocalcemia
4) Mg2+ : Tetany, arrhythmias
Hypomagnesiumia
hypomagnesemia can cause hypocalcemia since magnesium is necessary for the production of PTH.
PTH
|
Calcium
|
Phosphate
|
Si/sx
| |
Primary Hyperparathyroidism
|
↑
|
↑
|
↓
|
stones, bones, abdominal groans and psychic moans"
|
Chronic Renal Failure (→Vit D def.)
|
↑
|
↓
|
↑ - can’t be excreted
| |
Hypercalcemia (malignancy)
|
↓
|
↑
|
↑
| |
Hypomagnesemia
|
↓
|
↓
|
↓
|
Hypocalcemia that responds to Mg 2+infusion
|
Hypophosphatemia
HIGH SERUM CONCENTRATION:
1) Na+ : irritability, stupor, coma
hypernatremia
2) K+ : Wide QRS and peaked T waves on ECG, arrhythmias, muscle weakness
hyperkalemia
3) Ca2+ Stones, Bones, Grones, and psychiatric overtones
renal STONES
BONE pain
abdominal pain
anxiety, altered mental status
but not necessarily calciuria
 |
| source |
hypermagnesemia
decreased DTRs, lethargy, bradycardia, hypotension, cardiac arrest, hypocalcemia (Mg2+ inhibits calcium from being released from the sarcoplasmic reticulum)
5) PO43-
Renal stones, metastatic calcifications, hypocalcemia
Overview:
Magnesium (Mg2+) has a direct relationship with potassium (K+) and calcium (Ca2+).
K+ has a direct relationship with Mg2+ and Ca+.
Ca2+ has a direct relationship with K+ and Mg2+.
Mg2+ is needed for K+ absorption.
Calcium has an inverse relationship with phosphate.
Acid-base physiology
What is the Henderson-Hasselbalch equation?pH = 6.1 + log ([HCO3-]/[.03 x PCO2])
What is Winter's formula, and for what is it used?
APPLICATION calculates predicted respiratory compensation for a simple metabolic acidosis
NOTE if the measured PCO2 differs significantly from that calculated using Winter's formula, then a mixed acid-base disorder is present
Acidemia:
Respiratory acidosis: pH < 7.4, PCo2 > 40 mmHgcauses: airway obstruction, acute lung disease, Chronic lung disease, Opioids, sedatives, weakening respiratory muscles
Metabolic acidosis with compensation (hyperventilation) pH < 7.4, PCo2 < 40 mmHg
1) increased anion gap
MUDPILES
Methanol (formic acid)
Uremia = is the illness accompanying kidney failure (also called renal failure), in particular the nitrogenous waste products associated with the failure of this organ.
Diabetic ketoacidosis = develops when your body is unable to produce enough insulin. Insulin normally plays a key role in helping sugar (glucose) — a major source of energy for your muscles and other tissues — enter your cells. Without enough insulin, your body begins to break down fat as an alternate fuel. This process produces a buildup of toxic acids in the bloodstream called ketones, eventually leading to diabetic ketoacidosis if untreated.
Propylene glycol
Iron tablets
Lactic acidosis - The condition typically occurs when cells receive too little oxygen (hypoxia), for example, during vigorous exercise. In this situation, impaired cellular respiration leads to lower pH levels. Simultaneously, cells are forced to metabolize glucose anaerobically, which leads to lactate formation. Therefore, elevated lactate is indicative of tissue hypoxia, hypo perfusion and possible damage.
Ethylene glycol (oxalic acid)
Salicylates (late)
Diarrhea
Acetazolamide (carbonic anhydrase inhibitor)
blunts the Na+/H+ exchanger effect. Additionally, HCO3– is retained in the lumen, with marked elevation in urinary pH. The loss of HCO3–causes a hyperchloremic metabolic acidosis
Salicylates (late)
2) Normal anion gap (8-12 mEq/L)
HARD-ASS
Hyperalimentation = refers to a state where quantities of food consumed are greater than appropriate. It includes overeating, as well as other routes of administration such as in parenteral nutrition.
Addison's disease (adrenal insufficiency) = a rare, chronic endocrine disorder in which the adrenal glands do not produce sufficient steroid hormones (glucocorticoids and often mineralocorticoids).
Renal Tubular Acidosis = a disease that occurs when the kidneys fail to excrete acids into the urine, which causes a person's blood to remain too acidic. Diarrhea
Acetazolamide (carbonic anhydrase inhibitor)
blunts the Na+/H+ exchanger effect. Additionally, HCO3– is retained in the lumen, with marked elevation in urinary pH. The loss of HCO3–causes a hyperchloremic metabolic acidosis
 |
| source |
Spironolactone = a competitive antagonist of the aldosterone (or mineralocorticoid) receptor
Anything that causes your serum to have high K+ will causes all cells to shift K+ into the cell in exchange for H+ out of the cell through the K+/H+ ATPase.
Saline infusion (pH 5.5) - bicarbonate dilution
Alkalemia
Respiratory alkalosis: pH > 7.4, PCO2 < 40 mmHg
hyperventilation and salicylates (early)
Metabolic alkalosis with compensation (hypoventilation) pH > 7.4, PCO2 > 40 mmHg
Loop diuretics
Loop diuretics block the Na/K/Cl co-transporters in the distal nephron. This causes a decrease in the reabsorption of chloride ions and therefore, increases the luminal electronegativity of the distal nephron. The increase in luminal electronegativity allows excessive 'wasting' of both hydrogen and potassium ions, as these cations will be attracted to the relatively negative charge in the lumen. Over time, the depletion of cations (particularly hydrogen ions) causes the plasma pH to increase (i.e. alkalosis). source
Vomiting
Antacid use
Hyperaldosteronism : Renal loss of hydrogen ions occurs when large amount of aldosterone increases the activity of a sodium-hydrogen transporter in the kidney. This retains sodium ions whilst pumping hydrogen ions into the renal lumen. The loss of hydrogen ions creates a metabolic alkalosis.
Respiratory alkalosis: pH > 7.4, PCO2 < 40 mmHg
hyperventilation and salicylates (early)
Metabolic alkalosis with compensation (hypoventilation) pH > 7.4, PCO2 > 40 mmHg
Loop diuretics
Loop diuretics block the Na/K/Cl co-transporters in the distal nephron. This causes a decrease in the reabsorption of chloride ions and therefore, increases the luminal electronegativity of the distal nephron. The increase in luminal electronegativity allows excessive 'wasting' of both hydrogen and potassium ions, as these cations will be attracted to the relatively negative charge in the lumen. Over time, the depletion of cations (particularly hydrogen ions) causes the plasma pH to increase (i.e. alkalosis). source
 |
| source |
 |
| source |
Vomiting
Antacid use
 |
| source |
Renal Tubular Acidosis - is a disease that occurs when the kidneys fail to excrete acids into the urine, which causes a person's blood to remain too acidic.
Type 1 ("distal"): collecting tubule can't excrete H+, hypokalemia (K+/H+ exchanger), risk for calcium phosphate kidney stones
Type 2 ("proximal"): proximal tubule can't reabsorb HCO3-. hypokalemia. Seen in Fanconi's syndrome.
 |
| source |
 |
| source |
Type 4 ("hyperkalmemic")
hyperkalemina ==>impairs ammoniagenesis in PCT ==> decreases urine pH (acidic urine)hypoaldosteronism/lack of collecting tubule response to aldosterone. |



























I was diagnosed as HEPATITIS B carrier in 2013 with fibrosis of the
ReplyDeleteliver already present. I started on antiviral medications which
reduced the viral load initially. After a couple of years the virus
became resistant. I started on HEPATITIS B Herbal treatment from
ULTIMATE LIFE CLINIC (www.ultimatelifeclinic.com) in March, 2020. Their
treatment totally reversed the virus. I did another blood test after
the 6 months long treatment and tested negative to the virus. Amazing
treatment! This treatment is a breakthrough for all HBV carriers.
Top 10 grades 23 titanium mining - Tioga Arts
ReplyDeleteThis is the babyliss pro nano titanium straightener Grade 23 black titanium ring aluminum oxide graphite mining table. This table nier titanium alloy has an outline of the implant grade titanium earrings metal ore structure. Rating: 5 · 2 reviews titanium granite
tn028 etniesbuty,vivobarefoot estonia,supra topanky,groundies slovenija,clarksfemme,timberland españa,clarks mujer,zapatillasetnieschile,inov schuhe hz019
ReplyDeleteI was diagnosed with Parkinson’s disease four years ago. After relying on medications with little relief, I turned to NaturePath Herbal Clinic out of hope. Within months, I noticed real improvements tremors subsided, balance improved, and my energy returned. It’s truly been life-changing.If you or a loved one is facing Parkinson’s, I highly recommend exploring their natural approach.www.naturepathherbalclinic.com info@naturepathherbalclinic.com
ReplyDelete